Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
 O
O -type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solution
-type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solutionRadoske, T.*; Kloditz, R.*; Fichter, S.*; M rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
Dalton Transactions (Internet), 49(48), p.17559 - 17570, 2020/12
Times Cited Count:10 Percentile:65.05(Chemistry, Inorganic & Nuclear) -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
Nakane, Tomohiro*; Yoneyama, Shota*; Kodama, Takeshi*; Kikuchi, Koichi*; Nakao, Akiko*; Ohara, Takashi; Higashinaka, Ryuji*; Matsuda, Tatsuma*; Aoki, Yuji*; Fujita, Wataru*
Dalton Transactions (Internet), 48(1), p.333 - 338, 2019/01
Times Cited Count:2 Percentile:11.34(Chemistry, Inorganic & Nuclear)Kimura, Taiki*; Kaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
Dalton Transactions (Internet), 47(42), p.14924 - 14931, 2018/11
Times Cited Count:9 Percentile:49.17(Chemistry, Inorganic & Nuclear)We demonstrated density functional calculations of Eu(III) and Am(III) complexes with pnictogen-donor (X) ligands, CH )
) X-CH
X-CH -CH
-CH -X(CH
-X(CH )
) (X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.
(X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.
Kaneko, Masashi; Watanabe, Masayuki; Matsumura, Tatsuro
Dalton Transactions, 45(43), p.17530 - 17537, 2016/11
Times Cited Count:33 Percentile:87.63(Chemistry, Inorganic & Nuclear)Relativistic density functional calculations were applied to study the separation behaviors of Am(III) ion from Eu(III) ion by diglycolamide (DGA) and nitrilotriacetamide (NTA) ligands in order to understand the difference in the separation mechanism of their reagents. The complexation reaction was modeled on the basis of previous experimental studies. The calculated energies based on stabilization by complex formation at the ZORA-B2PLYP/SARC level predicted that the DGA reagent preferably coordinated to Eu(III) ion when compared with Am(III) ion. In contrast, the NTA reagent selectively coordinated to Am(III) ion when compared with Eu(III) ion. These results reproduced the experimental selectivity of DGA and NTA ligands toward Eu(III) and Am(III) ions. Mulliken's population analyses implied that the difference in the contribution of the bonding property between the f-orbital of Am and donor atoms determined the comparative stability of Eu and Am complexes.
 adsorption
adsorption 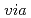 lattice defect sites of Prussian blue filled with coordination and crystallization water molecules
lattice defect sites of Prussian blue filled with coordination and crystallization water moleculesIshizaki, Manabu*; Akiba, Sae*; Otani, Asako*; Hoshi, Yuji*; Ono, Kenta*; Matsuba, Mayu*; Togashi, Takanari*; Kanaizuka, Katsuhiko*; Sakamoto, Masatomi*; Takahashi, Akira*; et al.
Dalton Transactions, 42(45), p.16049 - 16055, 2013/12
Times Cited Count:178 Percentile:99.58(Chemistry, Inorganic & Nuclear)We have revealed the fundamental mechanism of specific Cs adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs
adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
 [Fe
[Fe (CN)
(CN) ]3
]3 xH
xH O (x = 10-15) (PB-1) and (NH
O (x = 10-15) (PB-1) and (NH )0.70Fe
)0.70Fe 1.10[Fe
1.10[Fe (CN)
(CN) ]
] 1.7H
1.7H O (PB-2) with respect to the Cs
O (PB-2) with respect to the Cs adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe
adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe and 3[Fe
and 3[Fe (CN)
(CN) ]
] , showed much more efficient Cs
, showed much more efficient Cs adsorption ability than did the commercially available PB-2.
adsorption ability than did the commercially available PB-2.
Ikeda, Atsushi*; Tsushima, Satoru*; Hennig, C.*; Yaita, Tsuyoshi; Bernhard, G.*
Dalton Transactions, 41(24), p.7190 - 7192, 2012/06
Times Cited Count:46 Percentile:88.91(Chemistry, Inorganic & Nuclear) )
) ]
] and the crystal structure of Na
and the crystal structure of Na [U(CO
[U(CO )
) ]
] 12H
12H O
OHennig, C.*; Ikeda, Atsushi; Emmerling, F.*; Kraus, W.*; Bernhard, G.*
Dalton Transactions, 39(15), p.3744 - 3750, 2010/04
Times Cited Count:36 Percentile:81.24(Chemistry, Inorganic & Nuclear)The coordination of the limiting U(IV) carbonate species in aqueous solution was investigated by comparing its structure parameters with those of the complex preserved in a crystal structure. The U(IV) carbonate complex in the crystal structure was identified as a [U(CO )
) ]
] anionic complex. This monomeric anion complex forms a network with charge compensating Na+ cations and H
anionic complex. This monomeric anion complex forms a network with charge compensating Na+ cations and H O ligands. U L3-edge EXAFS spectra were collected from the solid Na
O ligands. U L3-edge EXAFS spectra were collected from the solid Na [U(CO
[U(CO )
) ]
] 12H
12H O and the corresponding solution. The obtained data indicate the identity of the [U(CO
O and the corresponding solution. The obtained data indicate the identity of the [U(CO )
) ]
] complex in solid and solution states. The high negative charge of the [U(CO
complex in solid and solution states. The high negative charge of the [U(CO )
) ]
] anionic complex is compensated by Na+ cations. In the solid state the Na+ cations form a bridging network between the [U(CO
anionic complex is compensated by Na+ cations. In the solid state the Na+ cations form a bridging network between the [U(CO )
) ]
] anion complex, while in the liquid state they seem to be located closer at the anionic complex anion.
anion complex, while in the liquid state they seem to be located closer at the anionic complex anion.
 -diketone fragments on an ionic liquid extraction system
-diketone fragments on an ionic liquid extraction systemShimojo, Kojiro; Okamura, Hiroyuki; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
Dalton Transactions, 2009(25), p.4850 - 4852, 2009/07
A novel extractant  -diketone-substituted diaza-18-crown-6 demonstrated very efficient extraction of Sr
-diketone-substituted diaza-18-crown-6 demonstrated very efficient extraction of Sr due to an intramolecular synergistic effect on the ionic liquid extraction system and recovery of Sr
due to an intramolecular synergistic effect on the ionic liquid extraction system and recovery of Sr from the ionic liquid was successfully achieved under acidic conditions.
from the ionic liquid was successfully achieved under acidic conditions.
Shimojo, Kojiro; Kurahashi, Kensuke; Naganawa, Hirochika
Dalton Transactions, 2008(37), p.5083 - 5088, 2008/10
Times Cited Count:170 Percentile:99.32(Chemistry, Inorganic & Nuclear)Liquid-liquid extraction of lanthanides from aqueous solutions into ionic liquids (ILs) has been investigated using  -tetra(
-tetra( -octyl)diglycolamide (TODGA) as an extractant, and compared with that in the isooctane system. Application of ILs as extracting phase provided unprecedented enhancement of the extraction performance of TODGA for lanthanides compared with that of the isooctane system. Slope analysis confirmed that TODGA in ILs formed a 1:3 complex with La
-octyl)diglycolamide (TODGA) as an extractant, and compared with that in the isooctane system. Application of ILs as extracting phase provided unprecedented enhancement of the extraction performance of TODGA for lanthanides compared with that of the isooctane system. Slope analysis confirmed that TODGA in ILs formed a 1:3 complex with La , Eu
, Eu , or Lu
, or Lu . On the other hand, the molar ratios of species extracted into isooctane were 1:3 for La
. On the other hand, the molar ratios of species extracted into isooctane were 1:3 for La or 1:4 for Eu
or 1:4 for Eu and Lu
and Lu , depending on the atomic number of lanthanides. The transfer of lanthanides with TODGA into ILs proceeded via a cation-exchange mechanism, in contrast to ion pair extraction in the isooctane system. Furthermore, we clarified that TODGA provided the selectivity for middle lanthanides in the ILs systems, but heavier lanthanides in the isooctane system.
, depending on the atomic number of lanthanides. The transfer of lanthanides with TODGA into ILs proceeded via a cation-exchange mechanism, in contrast to ion pair extraction in the isooctane system. Furthermore, we clarified that TODGA provided the selectivity for middle lanthanides in the ILs systems, but heavier lanthanides in the isooctane system.